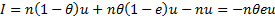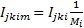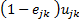Name of Lead Author: N. Hassoun
Organization: Binghamton University
Country: United States
Abstract
Understanding key technologies’ impact on the global burden of disease is essential for policy makers to extend access on important medicines to achieve Sustainable Development Goal 3 and fulfill everyone’s human right to health. This proposal aims to address the misalignment between the rights of inventors, trade rules, international human rights law, and public health in promoting access to important health technologies. It does so by providing a new dynamic model measuring key HIV/AIDS, malaria, and tuberculosis (TB) medicines’ effect on morbidity and mortality.
A comprehensive and accurate model of medicines’ global health impact is important for evaluating performance, setting targets, guiding the distribution of scarce health resources, and advancing access to affordable medicines. The Global Health Impact (GHI) model currently evaluates the global impact of medicines for HIV/AIDS, malaria, and TB and aggregates this information by company, drug, and disease as well as country. It estimates disease impact in the absence of treatment using data on drug effectiveness (or, barring that, efficacy), disease incidence, patient treatment coverage, and the global burden of disease that remains after treatment. We propose to expand this highly accurate, and innovative, metric to advance the measurement and understanding of pharmaceuticals impact in promoting global health. The preliminary version of the GHI model was recently published in PLoS One. The proposed methodology underlying the new version of the model is described in the section on implementation, evidence, and innovation below.
Making the new model and data on medicines’ global health impact available to researchers, policy-makers, consumers, companies, and other key stake-holders will increase their abilities to promote global health. This will provide states, non-governmental organizations, and companies with the means to promote new market strategies as well as innovative health policies that aim to provide worldwide equal access to medicine.
Submission
Advancing the Sustainable Development Goals and Human Rights to Health:
Evaluating Global Health Impact and Increasing Access to Essential Medicines
1. Overview
Understanding key technologies’ impact on the global burden of disease is essential for policy makers to extend access on important medicines to achieve Sustainable Development Goal 3 and fulfill everyone’s human right to health. This proposal aims to address the misalignment between the rights of inventors, trade rules, international human rights law, and public health in promoting access to important health technologies. It does so by providing a new dynamic model measuring key HIV/AIDS, malaria, and tuberculosis (TB) medicines’ effect on morbidity and mortality.
A comprehensive and accurate model of medicines’ global health impact is important for evaluating performance, setting targets, guiding the distribution of scarce health resources, and advancing access to affordable medicines. The Global Health Impact (GHI) model currently evaluates the global impact of medicines for HIV/AIDS, malaria, and TB and aggregates this information by company, drug, and disease as well as country [1-6]. It estimates disease impact in the absence of treatment using data on drug effectiveness (or, barring that, efficacy), disease incidence, patient treatment coverage, and the global burden of disease that remains after treatment [7-97]. We propose to expand this highly accurate, and innovative, metric to advance the understanding of pharmaceuticals’ impact in promoting everyone’s human right to health [65-68]. The preliminary version of the GHI model was recently published in PLoS One [1]. The proposed methodology underlying the new version of the model is described in the section on implementation, evidence, and innovation below. Making the new model and data on medicines’ global health impact available to researchers, policy-makers, consumers, companies, and other key stake-holders will increase their abilities to fulfill individuals’ human rights to health.. The model will also provide states, non-governmental organizations, and companies with the means to promote new market strategies and innovative health policies to help achieve Sustainable Development Goal 3.
2. Helping to Achieve Sustainable Development and Fulfill the Human Right to Health: Impact on Public Health and Policy Coherence
The GHI model can be used to help achieve Sustainable Development Goal 3 and fulfill individuals’ human rights to health [98-104]. It creates a common framework for judging health impact across a wide variety of interventions for multiple actors including private companies and international health organizations. It can thus help achieve universal access to quality essential health-care services as well as effective and affordable essential technologies. In particular, it can help address the epidemics of HIV/AIDS, malaria and TB. In doing so, it will also help reduce deaths of children under 5 years of age as malaria is a major contributor to these deaths. Moreover, the model can also be expanded to help address neglected tropical diseases as well as other communicable and non-communicable diseases.
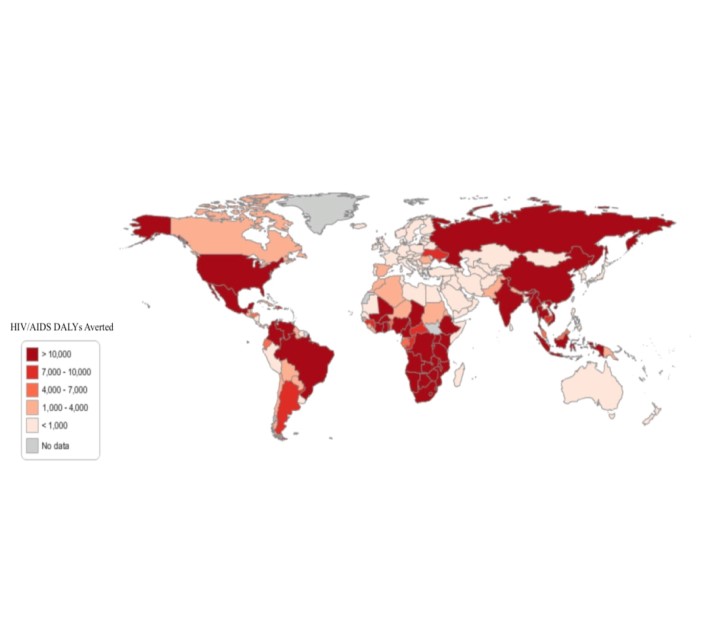
One way the model can be used to help address the epidemics of the diseases it evaluates is by highlighting the importance of paying attention not just to the burden of disease, but also its alleviation. Consider the need vs. impact graphs for HIV/AIDS from a preliminary version of the new model for 2010 (the methodology for the new model is described briefly in the appendix). The “Need” and “Impact” graphs below use Disability Adjusted Life Years (DALYs) as the basis for measuring need and impact. Globally, the data shows that there is a great amount of unmet need in the HIV/AIDS epidemic. Identifying current shortcomings is one step in identifying what can be done differently in order to address such shortcomings.
Need for and Impact of HIV/AIDS Medicines Around the World
Fig 2. Estimated Global Burden of Disease in the Absence of Treatment (in DALYs) for HIV/AIDS and Estimated Impact (in DALYS) for HIV/AIDS
While inadequate access to medicines for diseases such as HIV/AIDS receives significant media attention, understanding of the access to medicines issue is hampered because there is currently no systematic way to quantify efforts to address this problem. Without the ability to objectively, and accurately, measure and understand access inequality and efforts to improve it, this issue is difficult to prioritize from both the policy and innovation perspective. The proposed work to expand and utilize this model to evaluate the impact of various policies for promoting access to essential medicines will generate both data and a methodology for rating efforts to extend access on essential medicines that quantifies the effectiveness and impact of investments. It will do so using an analytical framework that can be applied to a broad array of other contexts where there are significant positive externalities of industry innovation and a “public goods” component to discovery. This framework can stimulate the access movement in three critical arenas: the market (allowing consumers to easily identify those companies whose products are having a large global health impact), policy creation (providing policy-makers the evidence-base needed to measure, understand, and respond to this issue), and industry (this metric will recognize and encourage investments that address this problem).
Our new model can help illustrate the impact of attempts to fulfill the human right to health and advance the Sustainable Development Goals. This data will provide some transparency into efforts to extend access to essential medicines, allowing policy makers to better target resources [6]. It will also provide useful information for customers (caregivers and patients), investors, pharmaceutical companies, and researchers interested in pharmaceutical research and development (R&D) and promoting global health. The new model can, for instance, help researchers to identify conditions that potentially influence drug impact so as to promote scientific progress through a new monitoring tool. Using the data we provide, researchers might evaluate the value of different investments or policy instruments’ effectiveness in terms of global health impact. They might consider the impact of medicines’ patent status (on or off-patent) on global health. Alternately, they can consider whether open access licensing was used, etc. They can also study the processes and structures that generate innovation and, so, good health outcomes.
Consider how the model can help set targets and monitor countries’ performance in utilizing the flexibilities to protect public health, and provide access to medicines for all, in the Doha Declaration on the World Trade Organization’s Agreement on Trade Related Aspects of Intellectual Property Rights’ (TRIPS’). Researchers can evaluate the impact of countries’ efforts to take advantage of these flexibilities and expand access to important health technologies for these diseases. Insofar as they are successful, these technologies’ impacts should increase in the model helping achieve universal access to quality essential health-care services and access to safe, effective, quality and affordable essential technologies for these diseases. So the model can help set targets and measure performance in fulfilling the human right to health and advancing the Sustainable Development Goals.
In summary, this ground-breaking research will show that it is possible to collect and analyze new and important data on access to essential medicines. It will spur knowledge generation by evaluating the health returns on investments in pharmaceutical research and development. The resulting rating system will provide useful information for evaluating the structures and processes that help create new knowledge and innovation. It will also show how such structures impact an important social outcome – in particular, global health. Although data alone will not solve any of the health problems people face, it can be used to help many people secure essential medicines that save millions of lives every year.
3. Implementation, Evidence, and Innovation
We have built a team of experts in health outcomes research, econometrics, biostatistics, and computer science and have an advisory board of representatives from academia and civil society from around the world to implement this project (global-health-impact.org). We are also working on outreach and soliciting feedback on the project from a variety of stakeholders. Once the scientific basis is well established, we will work on expanding our network to ensure that the model is used to evaluate policies and create incentives for a variety of agents to have a greater global health impact. Below is some information about our specific aims for developing the scientific basis for the model over the next few years.
The GHI model is currently the “gold standard” for evaluating the impact of key medicines for HIV/AIDS, malaria, and tuberculosis (TB) on disease burden in poor countries around the world [1-6]. Its methodology is significantly different than that embodied in other models as it estimates in a simple, transparent, and consistent way the health consequences of drugs for several leading diseases in global poverty areas [7-11]. Gathering data by company, drug, disease, and country into a single index, the original GHI model is able to estimate disease burden for any one or all conditions.
While the original GHI model is an excellent first step to understanding the impact of major pharmaceuticals on these diseases, it fails to estimate the burden of disease that occurs in the absence of treatment, the impact of drugs on this burden over time, or consider the contribution of generic firms to alleviating the burden. Here is a diagram illustrating the conceptual basis for the new proposed GHI model:
Fig 1. The New Conceptual Model
We assume that the global burden of disease that remains after treatment (in DALYs lost) is the average impact of an untreated or ineffectively treated case times the number of such cases. We also assume that treatment impact (DALYs saved) equals the number who are effectively treated times the average impact of an untreated or ineffectively treated case. We will use this fact and an estimate we derive of the number untreated or ineffectively treated (from data on treatment percentage and efficacy) to back out an estimate of the average impact of an untreated or ineffectively treated case. The average impact of an untreated or ineffectively treated case = Global Burden of Disease / (number untreated + the number ineffectively treated). Then using our estimate of the number who are effectively treated, we can calculate the impact of treatment. This is the average impact of an untreated or ineffectively treated case * the number of people who need a drug who are treated effectively [see appendix for further information on the model].
We will draw on the expertise of health outcomes researchers, pharmacologists, biostatisticians, and econometricians to construct, analyze, and validate this more sophisticated index that will measure health in the presence and absence of treatment for over time [105-126]. This new index will produce data on worldwide access to essential medicines that can be used to generate strategies for providing more equal access to these medicines and scientific innovation to address important global health problems. More generally, this information can help fulfill individuals’ human rights to health and achieve Sustainable Development Goal 3.
For further information about the preliminary models and the principles on which the contribution is based, see: [1-6, 127]. These contributions also detail important limitations of the models. Our analysis will not, for instance, perfectly capture the volume of each product used in each country. Where data is lacking, an estimate is provided based on the extent to which products are listed as first-line drugs for treating a disease. If 100 patients are treated in a country, and there are two first-line drugs, each drug is given credit for its potential impact on 50 patients. Our approach has the merit of allocating credit for being recognized as a first-line therapy for a given disease. Similarly, we will not attempt to determine what the incremental benefit of a drug is, relative to the next best therapy. Our analysis, in effect, indicates the impact of treatment including drugs in place of no treatment. Even with the inaccuracies that inevitably arise in this exercise, we believe that it is important to understand and illustrate what impact pharmaceuticals are having on global health and that this project can create incentives for promoting sustainable development and the human right to health [128]. Sensitivity analysis of the preliminary version suggest that many of the assumptions have little impact on the model [1].
The GHI model’s methodology is significantly different than that embodied in other models of disease burdens [7-11, 74-75]. The Futures Institute produces several dynamic models focused primarily on HIV/AIDS. Their AIDS Impact Model (AIM), for instance, looks at “the consequences of the HIV epidemic, including the number of people living with HIV, new infections, and AIDS deaths by age and sex; as well as the new cases of tuberculosis and AIDS orphans” [74]. Their Prevention of Mother-to-Child Transmission (PMTCT) model “evaluates the costs and benefits of intervention programs to reduce transmission of HIV from mother to child” including information on seven possible treatment regimens as well as other interventions [74]. Their Lives Saved Tool (LIST) considers the impact of different child health interventions on child mortality. Most models do not use DALYs, but focus on mortality rates, and so leave out a large component of interventions’ impacts.
The Futures Institute’s models rely on different kinds of information and make different assumptions than the new GHI model will employ. AIM assumes information “about the past and future course of adult HIV incidence and treatment coverage” as well as “the survival period from HIV infection to AIDS death, the age and sex distribution of new infections, and the perinatal transmission rate” [74]. Moreover, there are many additional assumptions in the demographic model upon which AIM draws that differ from the assumptions implicit in the new GHI model [74].
The GHI model has several advantages over alternative models. None of the other models combine – in a simple, transparent, consistent way – estimates of the death and disability saved by medicines for HIV/AIDS, malaria, and TB [7-11,74,75]. Because it uses the IHME’s DALY information, the new GHI model includes comparable estimates of the interventions’ impacts on disability as well as death across several diseases. The new model also includes many more interventions than most models and aggregates this information by company, drug, and disease as well as country.
High quality data on health impact will improve global governance for health, making it more transparent and accountable. The AIDS crisis revealed problems with global health structures that posed barriers to reducing infections. To address these problems, recent conceptions of development expressed, for instance, in the Paris Principles have emphasized the importance of achieving “measurable health improvements.” This aim is embodied in relatively recent global public/private partnerships advancing the global health agenda by employing performance-based mechanisms for allocating assistance. The Global Fund reports that it has helped save 17 million lives and the GAVI Alliance reports that it helped save 5.5 million. Comparative evaluation facilitates learning across programs and countries, and induces change: governments are much more likely to improve programs when the shortfalls are known and there are demonstrably better approaches. Other organizations can also use this data to expand access to essential medicines around the world.
Several things are necessary to ensure that the project promotes sustainable health outcomes. A sustainable funding stream would allow us to scale up the model even further and improve the scientific analysis behind the project. Expanding our network to partner with international institutions and governmental and non-governmental agencies working on promoting access to essential medicines would ensure that the data is presented in a way that is most useful for efforts to expand access.
4.Conclusion
The Sustainable Development Goals, in setting the post-2015 development agenda, are refocusing efforts on universal health coverage and access to essential medicines. As in other areas, information is the key to success in improving health outcomes and fulfilling individuals’ rights to health. A broad, accurate picture of what we have achieved in extending access on essential medicines is necessary to guide national and international organizations. The Global Burden of Disease project has, over the past 20 years, been immensely valuable in illuminating areas of need and it has had an undeniable influence on health spending. There is no similarly comprehensive effort to evaluate the impact of funding and interventions to improve global health. According to the WHO-WB framework for measuring UHC “the post-2015 agenda should address the unfinished agenda of the health-related MDGs as well as the emerging burden of chronic conditions and injuries (CCIs)” and this evaluation should eventually be extended to address the global priorities embodied in the Sustainable Development Goals. Ideally the evaluation would include preventative and diagnostic technologies as well as treatments for SDG priority health conditions [129-130].
There would moreover be multiple, important uses for such data among numerous stakeholders—ranging from international institutions like the World Health Organization to bilateral or multilateral health assistance organizations like The President’s Emergency Program on AIDS Relief and philanthropies like the Gates Foundation to national governments. Good data about effectiveness can help international and multilateral institutions (and other stakeholders) prioritize funding across countries, diseases and interventions. Country-level health systems similarly aim to allocate their resources, and secure new resources, to have a greater impact. Comprehensive data is important for evaluating performance, setting targets, and guiding the distribution of resources. Expanded models might complement existing work by global development agencies using tools like LiST to assess the impact of important health interventions [131]. If, for instance, countries can see how drugs impacts would change as they are made more accessible, that would help them decide whether to invest in efforts to lower prices or extend access more broadly in other ways. Moreover, impact assessments may help shape policies linked to broader macroeconomic and trade liberalization aspects of globalization and improve global health governance [132].
The High-Level Panel on the Post-2015 Development Agenda called for “a data revolution” to “help guide decision making, update priorities and ensure accountability” [133]. Good information systems are just as important to fulfilling individuals’ human rights to health as diagnostic tests are for identifying and treating disease. As the world sets its sights on achieving sustainable development with the Sustainable Development Goals, it is important to collect new data that can guide these efforts. Millions of lives hang in the balance.
Appendix, References and Bibliography
Appendix
More formally, consider a patient group identified by the IHME’s GBD data [63,77-78], for example, patients affected by a given disease indexed by j in a given country indexed by k. For this group, define as the average impact per untreated patient of the disease in terms of DALYs lost. Similarly, define the treated impact as
where
In effect,
is the effectiveness of the treatment, relative to the baseline of no treatment. For example, a drug that reduces DALYs lost by 95% would have
The DALYs observed within the patient group by the IHME’s GBD study are given by (1)
where
represents the proportion of infected persons who received treatment and n represents number of infected persons (i.e. the prevalence of disease times the population). (We have suppressed subscripts.) Assuming that treated and untreated individuals are similar, except for treatment, the DALYs that would occur in the absence of treatment is simply
(2)
Therefore the impact of treatment is given by
(3)
where this negative number represents a reduction in DALYs. Rearranging (1) we obtain
(1*)
Substituting (1’) into (3), we can write the impact of treatment as
(4)
The contribution of drug m to treatment regimen i with drugs is assumed to be an equal share of the regimen, and the impact of the drug is therefore simply given by
(5)
In effect, using this approach, we can identify the impact of treatment with a given regimen using (a) data from the IHME’s GBD study, (b) information on coverage of the patient population with a given regimen and (c) the effectiveness of the regimen compared to no treatment. Current treatments for HIV/AIDS, TB, and malaria are quite effective, and e may therefore typically be expected to be in the range [0.8, 1].
Coverage
is written as a stock, but in many cases treatment takes time, and so coverage should reflect the proportion of the patient population that is completing treatment. We will therefore adjust coverage by dividing it by the average duration of treatment if this number exceeds one year. Further details of the methodology specific to each disease and data sources are below.
Let’s use an example to illustrate how the impact score of a drug for one country can be calculated. Consider Artemether Lumefantrine (AL) in Bangladesh. For Bangladesh, 191,646 DALYs were lost due to malaria in 2010 [78]. About 92% of malaria in Bangladesh is Plasmodium falciparum malaria [79]. So the DALYs lost to Plasmodium falciparum malaria was 92% of 191,646, or 176,314. AL is used as the first-line drug for Plasmodium falciparum malaria in Bangladesh [79]. The treatment coverage for AL is 0.3% [80] and AL is efficacious in about 91.70% of cases [81]. The impact score of AL is calculated using the simplified formula
For Bangladesh, the DALYs saved by AL as a first-line drug is 176,314*0.3%*91.7%/(1-0.3%*91.7%)=515.10.
References
1. Hassoun, N. The Global Health Impact Index: promoting global health. PLoS ONE 2015 Dec; 10(12): 10.1371/journal.pone.0141374. Available: journals.plos.org/plosone/article?id=10.1371/journal.pone.0141374.
2. Hassoun, N. Modeling key malaria drugs’ impact on global health: a reason to invest in the Global Health Impact Index. Am J Trop Med Hyg. Forthcoming.
3. Hassoun, N. Global health impact: A basis for labeling and licensing campaigns? Dev World Bioeth 2012 Dec; 12(3): 121-134.
4. Hassoun, N. Rating efforts to extend access on essential medicines: increasing global health impact. In: Lenard P, Straehle C, editors. Health Inequalities and Global Justice. Edinburg: Edinburgh University Press; 2012. pp. 176-196.
5. Hassoun, N. Globalization, global justice and global health impact. Public Aff Q 2014 Jul; 28(3): 231-258. Available: http://paq.press.illinois.edu/28/3/index.html. Accessed 1 Jan 2015.
6. About the Index. 2014 [cited 29 Dec 2015]. In: Global Health Impact Organization Website [internet]. Global Health Impact. Available: www.global-health-impact.org/aboutindex.php. Accessed 29 Dec 2015.
7. Eisele, T.P., Larsen, D.A., Walker, N., Cibulskis, R.E., Yukich, J.O., Zikusooka, C.M., Steketee, R.W. Estimates of child deaths prevented from malaria prevention scale-up in Africa 2001-2010. Malar J 2012; 11: 93. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3350413/. Accessed 29 Dec 2015.
8. Galactionova, K., Tediosi, F., Savigny, D., Smith, T., Tanner, M. Effective coverage and systems effectiveness for malaria case management in Sub-Saharan African countries. PLoS One. 2015; 10(5): e0127818. Available: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0127818. Access 15 Sep 2015.
9. Bhatt, S., Weiss, D.J., Cameron, E., Bisanzio, D., Mappin, B., Dalrymple, U., Battle, K.E., Moyes, C.L., Henry, A., Eckhoff, P.A., Wenger, E.A., Briët, O., Penny, M.A., Smith, T.A., Bennett, A., Yukich, J., Eisele, T.P., Griffin, J.T., Fergus, C.A., Lynch, M., Lindgren, F., Cohen, J.M., Murray, C.L.J., Smith, D.L., Hay, S.I., Cibulskis, R.E., Gething, P.W. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015 Oct; 526(7572): 207-211.
10. Komatsu, R., Korenromp, E.L., Low-Beer, D., Watt, C., Dye, C., Steketee, R.W., Nahlen, B.L., Lyerla, R., Garcia-Calleja, J.M., Cutler, J., Schwartländer, B. Lives saved by global fund supported HIV/AIDS, tuberculosis and malaria programs: estimation approach and results between 2003 and end-2007. BMC Infect Dis Dec 2010; 10(109): 10.1186/1471-2334-10-109.
11. Bao, L. A new infectious disease model for estimating and projecting HIV/AIDS epidemics. Sex Transm Dis 2012 Dec; 88(Suppl_2): i58–i64. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512439/. Accessed 29 Dec 2015.
12. Malaria Treatment. Database: IndexMundi Data Portal [internet]. IndexMundi. Available: http://www.indexmundi.com/search.html?cx=partner-pub-5592729262323637%3Aj8a845havjo&cof=FORID%3A10&q=malaria+treatment&sa=Search&siteurl=www.indexmundi.com%2F&ref=www.indexmundi.com%2Fangola%2Fmalaria-treatment.html&ss=1783j236597j17. Accessed 29 Dec 2015.
13. World Health Organization. Global report on antimalarial drug efficacy and drug resistance: 2000-2010. Geneva: World Health Organization; 2010. Available: http://whqlibdoc.who.int/publications/2010/9789241500470_eng.pdf. Accessed 29 Dec 2015.
14. Priott, G., Kabakyenga, J., Pinoges, L., Ruiz, A., Eriksson, T., Coussement, F., Ngambe, T., Taylor, W.R., Perea, W., Guthmann, J.P., Olliaro, P., Legros, D. Artesunate and sulfadoxine-pyrimethamine combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Uganda: a randomized, double-blind, placebo-controlled trial. Trans R Soc Trop Med Hyg 2003 May; 97(3): 325-330. Available: http://www.ncbi.nlm.nih.gov/pubmed/15228253?dopt=Abstract. Accessed 29 Dec 2015.
15. Yavo, W., Faye, B., Kuete, T., Djohan, V., Oga, S.A., Kassi R.R., Diatta, M., Ama, M.V., Tine, R., Ndiaye, J.L., Evi, J.B., Same-Ekobo, A., Faye, O., Koné, M. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malar J 2011; 10: 198.
16. Tietche, F., Chelo, D., Mina Ntoto, N.K., Djoukoue, F.M., Hatz, C., Frey, S., Frentzel, A., Trapp, S., Zielonka, R., Mueller, EA. Tolerability and efficacy of a pediatric granule formulation of artesunate-mefloquine in young children from cameroon with uncomplicated falciparum malaria. Am J Trop Med Hyg 2010; 82(6): 1034-1040.
17. Tall, A., Rabarijaona L.P., Robert V., Bedja S.A., Ariey F. Randrianarivelojosia M. Efficacy of artesunate plus amodiaquine, artesunate plus sulfadoxine-pyrimethamine, and chloroquine plus sulfadoxine-pyrimethamine in patients with uncomplicated plasmodium falciparum in the Comoros Union. Acta Trop 2007; 102: 176-181.
18. The Four Artemisinin-Based Combinations (4ABC) Study Group. (A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med 2011; 8: 1-16.
19. Valecha, N., Phyo, A. P., Mayxay, M., Newton, P. N., Krudsood, S., Keomany, S., Khanthavong, M., Pongvongsa, T., Ruangveerayuth, R., Uthaisil, C., Ubben, D., Duparc, S., Bacchieri, A., Corsi, M., Rao, B. H. K., Bhattacharya, P. C., Dubhashi, N., Ghosh, S. K., Dev, V. Kumar, Ashwani Pukrittayakamee, S. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 2010; 5: 1-13.
20. Anvikar, A., Anupkumar R., Sharma, B., Shahi, B. H., Tyagi, P. K., Bose, T. K., Sharma, S. K. Srivastava, P., Srivastava, B., Kiechel, J. R., Dash, A. P., Valecha, N. Artesunate-amodiaquine fixed dose combination for the treatment of plasmodium falciparum malaria in India. Malar J 2012; 11: 97-104.
21. Sagara, I., Diallo, A., Kone, M., Coulibaly, M., Diawara, S.I., Guindo, O, Maiga, H., Niambele M. B., Sissoko, M., Dicko, A., Djimde, A., Doumbo, O. K. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated plasmodium falciparum malaria in Mali. Am J Trop Med Hyg 2008; 79: 655-61.
22. Zwang, J., Ashley, E. A., Karema, C., D'Alessandro, U., Smithuis, F., Dorsey, G., Janssens, B., Mayxay, M., Newton, P., Singhasivanon, P., Stepniewska, K., White, N. J., Nosten, F. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 2009; 4: 1-13.
23. World Health Organization. World malaria report 2011 annexes. Geneva: The World Health Organization; 2011. Available: http://www.who.int/malaria/world_malaria_report_2011/WMR2011_annexes_lowres.pdf. Accessed 29 Dec 2015.
24. World Health Organization. Data on the HIV/AIDS response: antiretroviral therapy coverage. Geneva: The World Health Organization; 2013. Available: http://apps.who.int/gho/data/node.main.574?lang=en. Accessed 29 Dec 2015.
25. Laurent, C., Kouanfack, C., Koulla-Shiro, S., Nkoué, N., Bourgeois, A., Calmy, A., Lactuock, B., Nzeusseu, V., Mougnutou, R., Peytavin, G., Liégeois, F., Nerrienet, E., Tardy, M., Peeters, M., Andrieux-Meyer, I., Zekeng, L., Kazatchkine, M., Mpoudi-Ngolé, E., Delaporte, E. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. The Lancet 2004; 364: 29-34.
26. Manosuthi, W.,Tantanathip, P., Prasithisirikul, W., Likanonsakul, S., Sungkanuparph, S. Durability of stavudine, lamivudine and nevirapine among advanced HIV-1 infected patients with/without prior co-administration of rifampicin: a 144-week prospective study. BMC Infect Dis 2008; 8: 136-142.
27. Munderi, P., Walker, A.S., Kityo, C., Babiker, A.G., Ssali, F., Reid, A., Darbyshire, J.H., Grosskurth, H., Mugyenyi, P., Gibb, D.M., Gilks, C.F., DART/NORA trial teams. Nevirapine/zidovudine/lamivudine has superior immunological and virological responses not reflected in clinical outcomes in a 48-week randomized comparison with abacavir/zidovudine/lamivudine in HIV-infected Ugandan adults with low CD4 cell counts. HIV Med 2010; 11: 334–344.
28. Gallant, J., Staszewski, S., Pozniak, A.L., DeJesus, E., Suleiman, J.M., Miller, M.D., Coakley, D.F., Lu, B., Toole, J.J., Cheng, A.K., 903 Study Group. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292: 191-201.
29. Annan, N., Torshie, Nelson, M., Mandalia, S., Bower, M., Gazzard, B. G., Stebbing, J. The nucleoside backbone affects durability of efavirenz- or nevirapine-based highly active antiretroviral therapy in antiretroviral-naive individuals. J Acquir Immune Defic Syndr 2009; 51: 140-6.
30. Kebba, A., Atwine, D., Mwebaze, R., Kityo, C., Nakityo, R., Peter, M. Therapeutic responses to AZT + 3TC + EFV in advanced antiretroviral naive HIV type 1-infected Ugandan patients. AIDS Res Hum Retroviruses 2002; 18: 1181-7.
31. Cohen, C., Molina, J.M., Cahn, P., Clotet, B., Fourie, J., Grinsztejn, B., Wu, H., Johnson, M.A., Saag, M., Supparatpinyo, K., Crauwels, H., Lefebvre, E., Rimsky, L.T., Vanveggel, S., Williams, P., Boven, K., ECHO Study Group, THRIVE Study Group. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naïve, HIV-1-infected patients: pooled results from the phase 3 double-blind, randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr 2012; 60: 33-42.
32. Sierra-Madero, J., Di Perri, G., Wood, R., Saag, M., Frank, I., Craig, C., Burnside, R., McCracken, J., Pontani, D., Goodrich, J., Heera, J., Mayer, H. Efficacy and safety of maraviroc versus efavirenz, both with zidovudine/lamivudine: 96-week results from the MERIT study. HIV Clin Trials 2010; 11: 125-32.
33. Gotuzzo, E., Markowitz, M., Ratanasuwan, W., Smith, G., Prada, G., Morales-Ramirez, J.O., Strohmaier, K.M., Lu, C., Bhanja, S., Nguyen, B.Y., Teppler, H., Protocol 004 Study Team. Sustained efficacy and safety of raltegravir after 5 years of combination antiretroviral therapy as initial treatment of HIV-1 infection: final results of a randomized, controlled, phase II study (Protocol 004). J Acquir Immune Defic Syndr 2012; 61: 73-7.
34. Markowitz, M., Nguyen, B.Y., Gotuzzo, E., Mendo, F., Ratanasuwan W., Kovacs, C., Prada, G., Morales-Ramirez, J.O., Crumpacker, C.S., Isaacs, R.D., Gilde, L.R., Wan, H., Miller, M.D., Wenning, L.A., Teppler, H., Protocol 004 Part II Study Team. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr 2007; 46: 125–33.
35. DeJesus, E., Rockstroh, J.K., Lennox, J.L., Saag, M.S., Lazzarin, A., Zhao, J., Wan, H., Rodgers, A.J., Walker, M.L., Miller, M., DiNubile, M.J., Nguyen, B.Y., Teppler, H., Leavitt, R.,Sklar, P, STARTMRK Investigators. Efficacy of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients: week-192 overall and subgroup analyses from STARTMRK. HIV Clin Trials 2012; 13: 228-32.
36. Gallant, J., DeJesus, E., Arribas, J.R., Pozniak, A.L., Gazzard, B., Campo, R.E., Lu, B., McColl, D., Chuck, S., Enejosa, J., Toole, J.J., Cheng, A.K., Study 934 Group. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006; 354: 251–60.
37. Lennox, J., DeJesus, E., Lazzarin, A., Pollard, R.B., Madruga, J.V., Berger, D.S., Zhao, J., Xu, X., Williams-Diaz, A., Rodgers, A.J., Barnard, R.J., Miller, M.D., DiNubile, M.J., Nguyen, B.Y., Leavitt, R., Sklar, P., STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, doubleblind randomised controlled trial. The Lancet 2009; 374: 796–806.
38. Daar, E., Tierney, C., Fischl, M.A., Sax, P.E., Mollan, K., Budhathoki, C., Godfrey, C., Jahed, N.C., Myers, L., Katzenstein, D., Farajallah, A., Rooney, J.F., Pappa, K.A., Woodward, W.C., Patterson, K., Bolivar, H., Benson, C.A., Collier, A.C., AIDS Clinical Trials Group Study A5202 Team. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154: 445–56.
39. Post, F., Moyle, G.J., Stellbrink, H.J., Domingo, P., Podzamczer, D., Fisher, M., Norden, A.G., Cavassini, M., Rieger, A., Khuong-Josses, M.A., Branco, T., Pearce, H.C., Givens, N., Vavro, C., Lim, M.L. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55: 49–57.
40. Puls, R., Puls, R.L., Srasuebkul, P., Petoumenos, K., Boesecke, C., Duncombe, C., Belloso, W.H., Molina, J.M., Li, L., Avihingsanon, A., Gazzard, B., Cooper, D.A., Emery, S., Altair Study Group. Efavirenz versus boosted atazanavir or zidovudine and abacavir in antiretroviral treatmentnaive, HIV-infected subjects: week 48 data from the Altair study. Clin Infect Dis 2010; 51: 855–64.
41. Campbell, T. B., Smeaton, L. M., Kumarasamy, N., Flanigan, T.,Klingman, K. L., Firnhaber, C., Grinsztejn, B., Hosseinipour, M. C., Kumwenda, J., Lalloo, U., Riviere, C., Sanchez, J., Melo, M., Supparatpinyo, K., Tripathy, S., Martinez, A. I., Nair, A., Walawander, A., Moran, L., Chen, Y., Snowden, W., Rooney, J. F., Uy, J., Schooley, R. T., De Gruttola, V., Hakim, J. G. Efficacy and safety of EFV of three antiretroviral regimens for initial treatment of HIV-1: randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9: e1001290.
42. Miró, J., Manzardo, C., Ferrer, E., Loncà, M., Guardo, A.C., Podzamczer, D., Domingo, P., Curran, A., Clotet, B., Cruceta, A., Lozano, F., Pérez, I., Plana, M., Gatell, J.M., Advanz-3 Study Group. Immune reconstitution in severely immunosuppressed antiretroviral-naive HIV-1-infected patients taking antiretroviral regimens based on efavirenz, lopinavir-ritonavir, and atazanavir-ritonavir: 48-week results of a randomized controlled trial. AIDS Res Hum Retroviruses 2010; 26: 747-57.
43. Molina, J., Cahn, P., Grinsztejn, B., Lazzarin, A., Mills, A., Saag, M., Supparatpinyo, K., Walmsley, S., Crauwels, H., Rimsky, L. T., Vanveggel, S., Boven, K. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active controlled trial. The Lancet 2011; 378: 238–46.
44. Landman, R., Poupard, M., Diallo, M., Ngom, G. N. F., Diakhate, N., Ndiaye, B., Toure, K. C., Trylesinski, A., Diop, H., Mboup, S., Koita, F., Delaporte, E., Benalycherif, A., Girard, P.M., Sow, P. S. Tenofovir-emtricitabineefavirenz in HIV-I-infected adults in Senegal: a 96-week pilot trial in treatment-naive patients. J Int Assoc Physicians AIDS Care 2009; 8: 379–84.
45. Rey, D., Hoen, B., Chavanet, P., Schmitt, M.P., Hoizey, G., Meyer, P., Peytavin, G., Spire, B., Allavena, C., Diemer, M., May, T., Schmit, J.L., Duong, M., Calvez, V., Lang, J.M. High rate of early virological failure with the once-daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother 2009; 63: 380–8.
46. Soriano, V., Arastéh, K., Migrone, H., Lutz, T., Opravil, M., Andrade-Villanueva, J., Antunes, F., Di Perri, G., Podzamczer, D., Taylor, S., Domingo, P., Gellermann, H., de Rossi, L., ARTEN investigators. Nevirapine versus atazanavir/ritonavir, each combined with tenofovir disoproxil fumarate/emtricitabine, in antiretroviral-naive HIV-1 patients: the ARTEN Trial. Antivir Ther 2011; 16: 339–48.
47. Lockman, S., Hughes, J., McIntyre, Y., Zheng, T., Chipato, F., Conradie, F., Sawe, A., Asmelash, M.C., Hosseinipour, L., Mohapi, E., Stringer, R. Mngqibisa, A. Siika, D. Atwine, J. Hakim, D. Shaffer, C. Kanyama, K. Wools-Kaloustian, R.A. Salata, E., Hogg, B., Alston-Smith, A., Walawander, E., Purcelle-Smith, S., Eshleman, J., Rooney, S., Rahim, J.W., Mellors, R.T., Schooley, J.S. Currier for the OCTANE A5208 Study Team. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 2010; 363: 1499–509.
48. Vallecillo, G., Domingo, P., Mallolas, J., Blanch, J., Ferrer, E., Cervantes, M., Pedrol, E., Knobel, H., Llibre, J.M. Evaluation of the safety and effectiveness of nevirapine plus coformulated tenofovir/emtricitabine as first-line therapy in routine clinical practice. AIDS Res Hum Retroviruses 2012; 28: 165-70.
49. Bunupuradah, T., Chetchotisakd, P., Ananworanich, J., Munsakul, W., Jirajariyavej, S., Kantipong, P., Prasithsirikul, W., Sungkanuparph, S., Bowonwatanuwong, C., Klinbuayaem, V., Kerr, S.J., Sophonphan, J., Bhakeecheep, S., Hirschel, B., Ruxrungtham, K., HIV STAR Study Group. A randomized comparison of second-line lopinavir/ritonavir monotherapy vs. tenofovir/lamivudine/lopinavir/ritonavir in patients failing NNRTI-regimens: the HIV STAR study. Antivir Ther 2012; 17: 1351-61.
50. Puthanakit, T., van der Lugt, J., Bunupuradah, T., Ananworanich, J., Gorowara, M., Phasomsap, C., Jupimai, T., Boonrak, P., Pancharoen, C., Burger, D., Ruxrungtham, K. Pharmacokinetics and 48 week efficacy of low-dose lopinavir/ritonavir in HIV-infected children. J Antimicrob Chemother 2009; 64: 1080-6.
51. Smith, K., Patel, P., Fine, D., Bellos, N., Sloan, L., Lackey, P., Kumar, P.N., Sutherland-Phillips, D.H., Vavro, C., Yau, L., Wannamaker, P., Shaefer, M.S., HEAT Study Team. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009; 23: 1547-56.
52. Saez-Llorens, X., Violari, A., Ndiweni, D., Yogev, R., Cashat, M., Wiznia, A., Chittick, G., Harris, J., Hinkle, J., Blum, M.R., Adda, N., Rousseau, F., FTC-203 Study Team. Long-term safety and efficacy results of once-daily emtricitabine-based highly active antiretroviral therapy regimens in human immunodeficiency virus-infected pediatric subjects. Pediatrics 2008; 121: e827-35.
53. Murphy, R., da Silva, B.A., Hicks, C.B., Eron, J.J., Gulick, R.M., Thompson, M.A., McMillan, F., King, M.S., Hanna, G.J., Brun, S.C. Seven-year efficacy of a lopinavir/ritonavir-based regimen in antiretroviral-naïve HIV-1-infected patients. HIV Clin Trials 2008; 9: 1-10.
54. Echeverría, P., Negredo, E., Carosi, G., Gálvez, J., Gómez, J.L., Ocampo, A., Portilla, J., Prieto, A., López, J.C., Rubio, R., Mariño, A., Pedrol, E., Viladés, C., del Arco, A., Moreno, A., Bravo, I., López-Blazquez, R., Pérez-Alvarez, N., Clotet, B. Similar antiviral efficacy and tolerability between efavirenz and lopinavir/ritonavir, administered with abacavir/lamivudine (Kivexa), in antiretroviral-naïve patients: a 48-week, multicentre, randomized study (Lake Study). Antiviral Res 2010; 85: 403-8.
55. Pensi, T. Fixed dose combination of lamivudine, stavudine and nevirapine in the treatment of pediatric HIV infection: a preliminary report. Indian Pediatr 2007; 44: 519-21.
56. Frange P., Briand, N., Avettand-fenoel, V., Veber, F., Moshous, D., Mahlaoui, N., Rouzioux, C., Blanche, S., Chaix, M.L. Lopinavir/ritonavir-based antiretroviral therapy in human immunodeficiency virus type 1-infected naive children: rare protease inhibitor resistance mutations but high lamivudine/emtricitabine resistance at the time of virologic failure. Pediatr Infect Dis J 2011; 30: 684-8.
57. World Health Organization. Antiretroviral medicines in low- and middle-income countries: usage in 2010 with global and regional demand forecast for 2011–2012. Geneva: The World Health Organization; 2013. Available: http://www.who.int/hiv/pub/amds/2013forecast_report/en/. Accessed 29 Dec 2015.
58. World Health Organization. Data for global tuberculosis control. Geneva: The World Health Organization. Available: http://who.int/tb/country/data/download/en/index.html. Accessed 29 Dec 2015.
59. World Health Organization. Global tuberculosis control 2011. Geneva: The World Health Organization; 2011. Available: http://apps.who.int/iris/bitstream/10665/44728/1/9789241564380_eng.pdf. Accessed 29 Dec 2015.
60. World Health Organization. Multidrug-resistant tuberculosis 2013 Update. Geneva: The World Health Organization; 2013. Available: http://www.who.int/tb/challenges/mdr/MDR_TB_FactSheet.pdf. Accessed 29 Dec 2015.
61. World Health Organization. 2007-2008 XDR & MDR Tuberculosis global response plan. Geneva: The World Health Organization; 2007. Available: http://www.who.int/tb/challenges/xdr/xdr_mdr_factsheet_2007_en.pdf. Accessed 29 Dec 2015.
62. World Health Organization. (2013). Global tuberculosis report 2013. Geneva: The World Health Organization; 2013. Available: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1. Accessed 29 Dec 2015.
63. Institute for Health Metrics and Evaluation. The global burden of disease: generating evidence: guiding policy. Seattle: University of Washington; 2013. Available: http://www.healthdata.org/sites/default/files/files/policy_report/2013/GBD_GeneratingEvidence/IHME_GBD_GeneratingEvidence_FullReport.pdf. Accessed 29 Dec 2015.
64. World Health Organization. Global price reporting mechanism for HIV, tuberculosis and malaria. In: World Health Organization Website [internet]. Geneva: World Health Organization; 2016. Available: http://www.who.int/hiv/amds/gprm/en/. Accessed 1 Jan 2016.
65. Silverman, E. A. New index measures impact pharma has on infectious diseases. The Wall Street Journal Pharmalot. 23 Jan 2015. Available: http://blogs.wsj.com/pharmalot/2015/01/23/a-new-index-measures-impact-pharma-has-on-infectious-diseases/. Accessed 29 Dec 2015.
66. Prakash N. SUNY Professor Indexes Pharma Companies' Impact. POLITICO New York beta. 23 Jan 2015. Available: http://www.capitalnewyork.com/article/albany/2015/01/8560712/suny-professor-indexes-pharma-companies-impact. Accessed 29 Dec 2015.
67. Gorenstein D. New effort ranks drugmakers by impact. American Public Media Marketplace: Health Care. 23 Jan 2015. Available: http://www.marketplace.org/topics/health-care/new-effort-ranks-drug-makers-impact. Accessed 29 Dec 2015.
68. Berman J. Web Tool Tracks Locations of Vital Drugs' Biggest Impact. Voice of America Health. 15 Dec 2015. Available: http://www.voanews.com/content/new-web-tool-identifies-drug-companies-helping-poor/3104287.html. Accessed 29 Dec 2015.
69. UN Secretary-General’s Independent Expert Advisory Group on a Data Revolution for Sustainable Development. A world that counts: mobilising the data revolution for sustainable development. November 2014. Available: http://www.undatarevolution.org/wp-content/uploads/2014/12/A-World-That-Counts2.pdf. Accessed 29 Dec 2015.
70. World Bank Group and International Monetary Fund. Global monitoring report 2015/2016: development goals in an era of demographic change. Advance Edition. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2016. p. 107, Box 2.3. Available: http://pubdocs.worldbank.org/pubdocs/publicdoc/2015/10/503001444058224597/Global-Monitoring-Report-2015.pdf. Accessed 29 Dec 2015.
71. Trouiller, P., Torreele, E., Olliaro, P., White, N., Foster, S., Wirth, D., Pécoul, B. Drugs for neglected diseases: a failure of the market and a public health failure? Trop Med Int Health 2002; 6: 945-51.
72. Chatterjee, S., Zheng Y. A survey on consumers’ willingness to purchase things with a Global Health Impact label. Paper under preparation. Binghamton University, 2014.
73. World Health Organization. Public health-innovation and intellectual property rights: report of the commission on intellectual property rights, innovation and public health. Geneva: The World Health Organization; 2006. Available: http://www.who.int/intellectualproperty/documents/thereport/ENPublicHealthReport.pdf. Accessed 29 Dec 2015.
74. Futures Group. 2015 [cited 29 Dec 2015]. Spectrum. In: Futures Group Website, Resources, Software/Models [internet]. Futures Group Global. Available: http://futuresgroup.com/resources/software_models/spectrum. Accessed 29 Dec 2015.
75. Stover, J. AIM: a computer program for making HIV/AIDS projections and examining the demographic and social impacts of AIDS. Washington, DC: Futures Group International, United States Agency of International Development Health Policy Initiative; 2009. pp. 4-5. Available: http://www.healthpolicyinitiative.com/Publications/Documents/1253_1_AimManE.pdf. Accessed 29 Dec 2015.
76. Access to Medicine Index. The access to medicine index 2012. Haarlem: Access to Medicine Foundation, 2012. Available: http://www.accesstomedicineindex.org/sites/2015.atmindex.org/files/2012-access-to-medicine-index-clickable.pdf. Accessed 29 Dec 2015.
77. Murray, C. J. L., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., Ezzati, M., Shibuya, K., Salomon, J. A., Abdalla, S., Aboyans, V., Abraham, J., Ackerman, I., Aggarwal, R., Ahn, S. Y., Ali, M. K., AlMazroa, M. A., Alvarado, M., Anderson, H. R., Anderson, L. M., Andrews, K. G., Atkinson, C., Baddour, L. M., Bahalim, A. N., Barker-Collo, S., Barrero, L. H., Bartels, D. H., Basáñez, M., Baxter, A., Bell, M. L., Benjamin, E. J., Bennett, D., Bernabé, E., Bhalla, K., Bhandari, B., Bikbov, B., Abdulhak, A., Birbeck, G., Black, J. A., Blencowe, H., Blore, J. D., Blyth, F., Bolliger, I., Bonaventure, A., Boufous, S., Bourne, R., Boussinesq, M., Braithwaite, T., Brayne, C., Bridgett, L., Brooker, S., Brooks, P., Brugha, T. S., Bryan-Hancock, C., Bucello, C., Buchbinder, R., Buckle, G., Budke, C. M., Burch, M., Burney, P., Burstein, R., Calabria, B., Campbell, B., Canter, C. E., Carabin, H., Carapetis, J., Carmona, L., Cella, C., Charlson, F., Chen, H., Cheng, A. T., Chou, D., Chugh, S. S., Coffeng, L. E., Colan, S. D., Colquhoun, S., Colson, K. E., Condon, J., Connor, M. D., Cooper, L. T., Corriere, M., Cortinovis, M., de Vaccaro, K. C., Couser, W., Cowie, B. C., Criqui, M. H., Cross, M., Dabhadkar, K. C., Dahiya, M., Dahodwala, N., Damsere-Derry, J., Danaei, G., Davis, A., Leo, D. D., Degenhardt, L., Dellavalle, R., Delossantos, A., Denenberg, J., Derrett, S., Des Jarlais, D. C., Dharmaratne, S. D., Dherani, M., Diaz-Torne, C., Dolk, H., Dorsey, E. R., Driscoll, T., Duber, H., Ebel, B., Edmond, K., Elbaz, A., Ali, S. E., Erskine, H., Erwin, P. J., Espindola, P., Ewoigbokhan, S. E., Farzadfar, F., Feigin, V., Felson, D. T., Ferrari, A., Ferri, C. P., Fèvre, E. M., ; Finucane, M. M., Flaxman, S., Flood, L., Foreman, K., Forouzanfar, M. H., Fowkes, F. G. R., Fransen, M., Freeman, M. K., Gabbe, B. J., Gabriel, S. E., Gakidou, E., Ganatra, H. A., Garcia, B., Gaspari, F., Gillum, R. F., Gmel, G., Gonzalez-Medina, D., Gosselin, R., Grainger, R., Grant, B., Groeger, J., Guillemin, F., Gunnell, D., Gupta, R., Haagsma, J., Hagan, H., Halasa, Y. A., Hall, W., Haring, D., Haro, J. M., Harrison, J. E., Havmoeller, R., Hay, Roderick J. H. H., Hill, C., Hoen, B., Hoffman, H., Hotez, P. J., Hoy, D., Huang, J. J., Ibeanusi, S. E., Jacobsen, K. H., James, Spencer L., Jarvis, D., Jasrasaria, R., Jayaraman, S., Johns, N., Jonas, Jost B., Karthikeyan, G., Kassebaum, N., Kawakami, N., Keren, A., Khoo, J., King, C. H., Knowlton, L. M., Kobusingye, O., Koranteng, A., Krishnamurthi, R., Laden, F., Lalloo, R., Laslett, L. L., Lathlean, T., Leasher, J. L., Lee, Y. Y., Leigh, J., Levinson, D., Lim, S. S., Limb, E., Lin, J. K., Lipnick, M., Lipshultz, S. E., Liu, W., Loane, M., Ohno, S. L., Lyons, R., Mabweijano, J., MacIntyre, M. F., Malekzadeh, R., Mallinger, L., Manivannan, S., Marcenes, W., March, L., Margolis, D. J., Marks, G. B., Marks, R., Matsumori, A., Matzopoulos, R., Mayosi, B. M., McAnulty, J. H., McDermott, M. M., McGill, N., McGrath, J., Medina-Mora, M. E., Meltzer, M., Memish, Z. A., Mensah, G. A., Merriman, T. R., Meyer, A., Miglioli, V., Miller, M., Miller, T. R., Mitchell, P. B., Mock, C., Mocumbi, A. O., Moffitt, T. E., Mokdad, A. A., Monasta, L., Montico, M., Moradi-Lakeh, M., Moran, A., Morawska, L., Mori, R., Murdoch, M. E., Mwaniki, M. K., Naidoo, K., Nair, M. N., Naldi, L., Narayan, K.M.V., Nelson, P. K., Nelson, R. G., Nevitt, M. C., Newton, C. R., Nolte, S., Norman, P., Norman, R., O'Donnell, M., O'Hanlon, S., Olives, C., Omer, S. B., Ortblad, K., Osborne, R., Ozgediz, D., Page, A., Pahari, B., Pandian, J. D., Rivero, A. P., Patten, S. B., Pearce, N., Padilla, R. P., Perez-Ruiz, F., Perico, N., Pesudovs, K., Phillips, D., Phillips, M. R., Pierce, K., Pion, S., Polanczyk, G. V., Polinder, S., Pope, C. A., Popova, S., Porrini, E., Pourmalek, F., Prince, M., Pullan, R. L., Ramaiah, K. D., Ranganathan, D., Razavi, H., Regan, M., Rehm, J. T., Rein, D. B., Remuzzi, G., Richardson, K., Rivara, F. P., Roberts, T., Robinson, C., De Leòn, F. R., Ronfani, L., Room, R., Rosenfeld, L. C., Rushton, L., Sacco, R. L., Saha, S., Sampson, U., Sanchez-Riera, L., Sanman, E., Schwebel, D. C., Scott, J. G., Segui-Gomez, M., Shahraz, S., Shepard, D. S., Shin, H., Shivakoti, R., Silberberg, D., Singh, D., Singh, G., Singh, J.A., Singleton, J., Sleet, D. A., Sliwa, K., Smith, E., Smith, J. L., Stapelberg, N.J.C., Steer, A., Steiner, T., Stolk, W. A., Stovner, L. J., Sudfeld, C., Syed, S., Tamburlini, G., Tavakkoli, M., Taylor, H. R., Taylor, J. A., Taylor, W. J.,Thomas, B., Thomson, W. M.,Thurston, G. D., Tleyjeh, I. M., Tonelli, M., Towbin, J. A., Truelsen, T., Tsilimbaris, M. K., Ubeda, C., Undurraga, E. A., van der Werf, M. J., van Os, J., Vavilala, M. S., Venketasubramanian, N., Wang, M., Wang, W., Watt, K., Weatherall, D. J., Weinstock, M. A.,Weintraub, R., Weisskopf, M. G., Weissman, M. M., White, R. A., Whiteford, H., Wiebe, N., Wiersma, S.T., Wilkinson, J. D., Williams, H.C., Williams, S.R.M., Witt, E.,Wolfe, F.,Woolf, A. D., Wulf, S., Yeh, P. H., Zaidi, A. K. M., Zheng, Z., Zonies, D., Lopez, A.D. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2013; 380: 2197-2223.
78. Global Health Data Exchange (GHDx). Country Profiles. Global Burden of Disease Study 2010 (GBD 2010) Results 1990-2010. Seattle: Institute for Health Metrics and Evaluation. Available: http://ghdx.healthmetricsandevaluation.org/country_profiles. Accessed 29 Dec 2015.
79. World Health Organization. World malaria report 2013 country profiles. Geneva: World Health Organization; 2013. Available: http://www.who.int/malaria/publications/world_malaria_report_2013/wmr2013_country_profiles.pdf?ua=1. Accessed 29 Dec 2015.
80. About UNICEF data and analytics. 2015. In: UNICEF Data Website [internet]. UNICEF. Available: http://www.data.unicef.org/indexd0c9.html?section=unicef_aboutus. Accessed 29 Dec 2015.
81. World Health Organization. Global report on antimalarial drug efficacy and drug resistance: 2000-2010. Geneva: World Health Organization; 2010. p. 98. Available: http://whqlibdoc.who.int/publications/2010/9789241500470_eng.pdf. Accessed 29 Dec 2015.
82. World Health Organization, UNICEF, UNAIDS. Global Update on HIV Treatment 2013: Results, Impact, and Opportunities. Geneva: The World Health Organization; 2013.
83. Meeting report: joint WHO/UNAIDS Annual Consultation with Pharmaceutical Companies and Stakeholders on Forecasting Global Demand of Antiretroviral Drugs for 2013-2016. 25-26 November, 2013, Geneva, Switzerland. Geneva: The World Health Organization; 2014.
84. Pan American Health Organization, World Health Organization Regional Office for the Americas. Antiretroviral Treatment in the Spotlight: A Public Health Analysis in Latin America and the Caribbean. Washington, D.C.: Regional Office for the Americas of the World Health Organization; 2014.
85. Disease Tutorials: HIV Tutorial 2-f. Survival Time on ART. 2015 [cited 29 Dec 2015]. In: Institute for Disease Modeling Website [internet]. Intellectual Ventures Property Holdings, LLC. Available: http://idmod.org/idmdoc/Content/EMOD/DiseaseTutorials/STI_and_HIV_Tutorials/2f_Survival_Time_on_ART.htm. Accessed 29 Dec 2015.
86. Delva, W. Coverage strategies: insights from population simulation models. International center for reproductive health and South African center for epidemiological modelling and analysis. In: Center for Effective Global Action, University of California, Berkeley Website [internet]. Available: http://cega.berkeley.edu/assets/cega_events/33/1.11_Coverage_Strategies_Delva.pdf. Accessed 29 Dec 2015.
87. Gadpayle, A.K., Kumar, N., Duggal, A., Rewari, B.B., Ravi, V. Survival trend and prognostic outcome of AIDS patients according to age, sex, stages, and mode of transmission – A retrospective study at ART centre of a tertiary care hospital. JIACM 2012; 13(4): 291-8. Available: http://medind.nic.in/jac/t12/i4/jact12i4p291.pdf. Accessed 29 Dec 2015.
88. World Health Organization. Technical report: Access to Antiretroviral Drugs in low- and middle- income countries. Geneva: World Health Organization; 2014. Available: http://apps.who.int/iris/bitstream/10665/128150/1/9789241507547_eng.pdf?ua=1&ua=1. Accessed 1 Jan 2016.
89. Waning, B., Diedrichsen, E., Moon, S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc 2010 Sep 14; 13: 35. Available: http://www.ncbi.nlm.nih.gov/pubmed/20840741. Accessed 15 Sep 2015.
90. World Health Organization. World malaria report 2012. Geneva: The World Health Organization; 2012. Available: http://www.who.int/malaria/publications/world_malaria_report_2012/en/. Accessed 29 Dec 2015.
91. Kedir, A.A., Desta, A., Fesseha, G. Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. J AIDS Clin Res 2014; 5(3): 289. Available: http://www.omicsonline.org/open-access/factors-affecting-survival-of-hiv-positive-children-taking-antiretroviral-therapy-at-adama-referral-hospital-and-medical-college-ethiopia-2155-6113.1000289.pdf. Accessed 29 Dec 2015.
92. Ayalew, J., Moges, H., sahu, O., Worku, A. Identifying factors related to the survival of aids patients under the follow-up of antiretroviral therapy (ART): the case of South Wollo. International Journal of Data Envelopment Analysis and *Operations Research* 2014 1(2): 21-27. Available: http://pubs.sciepub.com/ijdeaor/1/2/2/. Accessed 29 Dec 2015.
93. Cornell, M., Schomaker, M., Garone, D.B., Giddy, J., Hoffmann, C.J., Lessells, R., Maskew M., Prozesky H., Wood R., Johnson L.F., Egger M., Boulle A., Myer L., International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration. Gender differences in survival among adult patients starting antiretroviral therapy in south africa: a multicentre cohort study. PLoS Medicine 2012; 9(9): e1001304.
94. Taylor-Smith, K., Tweya, H., Harries, A., Schoutene, E., Jahn, A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J 2010; 22(2): 49–56. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3345762/. Accessed 29 Dec 2015.
95. Mitnick, C., Sonya, S., Kwonjune, J. S., Rich, M. L., Atwood, S. S., Furin, J. J., Fitzmaurice, G. M., Alcantara Viru, F. A., Appleton, S. C., Bayona, J. N., Bonilla, C. A., Chalco, K., Choi, S., Franke, M. F., Fraser, H. S. F., Guerra, D., Hurtado, R. M., Darius, J., Joseph, K., Llaro, K., Mestanza, L., Mukherjee, J. S., Muñoz, M., Palacios, E., Sanchez, E., Sloutsky, A., Becerra, M. C. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med 2008; 359: 563-574.
96. Mphahlele, M., Syre, H., Valvatne, H., Stavrum, R., Mannsåker, T., Muthivhi, T., Weyer, K., Fourie, P.B., Grewal, H.M. Pyrazinamide resistance among South African multidrug-resistant mycobacterium tuberculosis isolates. J Clin Microbiol 2008; 46: 3459-3464.
97. Lafferty, J., Wasserman, L. Statistical analysis of semi-supervised regression. Carnegie Mellon University Research Showcase; 2007. Available: http://repository.cmu.edu/cgi/viewcontent.cgi?article=2031&context=compsci. Accessed 29 Dec 2015.
98. Committee on Economic, Social and Cultural Rights (CESCR). 1990. General Comment 3: The Nature of States Parties’ Obligations, Fifth Session, UN Doc. E/1991/23, annex III at 86(1991) Reprinted in Compilation of General Comments and General Recommendations Adapted by Human Rights Treaty Bodies, UN Doc. HRI/Gen/I/Rev. 6 at 62 (2003). http://www1.umn.edu/humanrts/gencomm/epcomm3.htm.
99. Gostin, Lawrence, et al. 2012. Towards a Framework Convention on Global Health. Bulletin of the World Health Organization.http://www.who.int/bulletin/volumes/91/10/12-114447/en/.
100. Gostin, Lawrence. 2001. The Human Right to Health: A Right to the ‘Highest Attainable Standard of Health. The Hastings Center Report 31 (2): 29-30.
101. United Nations (UN). 1966. Universal Declaration of Human Rights (UNDHR). International Covenant on Economic, Social and Cultural Rights Office of the High Commissioner of Human Rights. http://www.ohchr.org/EN/ProfessionalInterest/Pages/CESCR.aspx.
102. Ruggie, John. 2008. Protect, Respect and Remedy: A Framework for Business and Human Rights Report of the Special Representative of the Secretary-General on the Issue of Human Rights and Transnational Corporations and Other Business Enterprises, Human Rights Council 8 (3). P. 18.
103. Hunt, Paul. 2008. Human Rights Guidelines for Pharmaceutical Companies in Relation to Access to Medicines. United Nations (August 11)
104. Lee, Joo Young and Paul Hunt. 2012. Human Rights Responsibilities of Pharmaceutical Companies in Relation to Access to Medicines. Journal of Law Medicine & Ethics 40 (2): 225.
105. Zhou, Z.H., Li, M. 2005. Semi-supervised regression with co-training. In: International Joint Conferences on Artificial Intelligence 2005 Proceedings Website [internet]. pp. 908-913. Available: http://www.ijcai.org/search.php. Accessed 29 Dec 2015.
106. World Bank Group and International Monetary Fund. Global monitoring report 2015/2016: development goals in an era of demographic change. Advance Edition. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2016. Available: http://pubdocs.worldbank.org/pubdocs/publicdoc/2015/10/503001444058224597/Global-Monitoring-Report-2015.pdf. Accessed 29 Dec 2015.
107. Goal: Achieve universal primary education. UNICEF Millenium Development Goals Website [internet]. Available: http://www.unicef.org/mdg/index_education.htm. Accessed 29 Dec 2015.
108. Goal: Promote gender equality and empower women. UNICEF Millenium Development Goals Website [internet]. Available: http://www.unicef.org/mdg/index_genderequality.htm. Accessed 29 Dec 2015.
109. Cutler, D., Lleras-Muney, A. Policy Brief #9: Education and Health. Mar 2007 [cited 29 Dec 2015]. In: The University of Michigan National Poverty Center Website. Ann Arbor: The University of Michigan. Available: http://www.npc.umich.edu/publications/policy_briefs/brief9/. Accessed 29 Dec 2015.
110. Health and Academics. 1 Sep 2015 [cited 29 Dec 2015]. Centers for Disease Control and Prevention Website [internet]. Atlanta: U.S. Department of Health & Human Services. Available: http://www.cdc.gov/HealthyYouth/health_and_academics/. Accessed 29 Dec 2015.
111. World Bank Group and International Monetary Fund. Global monitoring report 2015/2016: development goals in an era of demographic change. Advance Edition. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2016. pp. 89-93. Available: http://pubdocs.worldbank.org/pubdocs/publicdoc/2015/10/503001444058224597/Global-Monitoring-Report-2015.pdf. Accessed 29 Dec 2015.
112. Lochner, L. Non-productive benefits of education: crime, health, and good citizenship. NBER Working Paper 16722. Cambridge, MA: National Bureau of Economic Research, Inc.; 2011. Available: http://www.nber.org/papers/w16722.pdf. Accessed 29 Dec 2015.
113. Alderman, H., Bleakley, H. Child health and educational outcomes. In Glewwe, P., editor. Education policy in developing countries. Chicago: The University of Chicago Press; 2014. pp. 107-136.
114. Strickland, B. Designing effective education programs for school health in developing countries compendium. United States Agency of International Development Educational Quality Improvement Program; 2011. Available: http://www.equip123.net/docs/E1-FP_Health_Comp_Web.pdf. Accessed 29 Dec 2015.
115. How We Work. 2015 [cited 29 Dec 2015]. In: Save the Children Organization Website [internet]. Fairfield: Save the Children. Available: http://www.savethechildren.org/site/c.8rKLIXMGIpI4E/b.6196517/k.9825/How_We_Work.htm. Accessed 29 Dec 2015.
116. Johnson & Johnson. 28 Dec 2015 [cited 29 Dec 2015]. In: Johnson & Johnson Company Website. Johnson & Johnson Services, Inc. Available: http://www.jnj.com/. Accessed 29 Dec 2015.
117. PHARMACEUTICALS: Facts, Policies and NCSL Resources. Nov 2015 [cited 29 Dec 2015]. In: National Conference of State Legislatures Website [internet]. Denver: National Conference of State Legislatures Website. Available: http://www.ncsl.org/research/health/pharmaceuticals-facts-policies-and-ncsl-resources.aspx. Accessed 29 Dec 2015.
118. Country Reports. 2015 [cited 29 Dec 2015]. In PMLiVE Website [internet]. PMGroup Worldwide Ltd. Available: http://www.pmlive.com/intelligence/country_reports. Accessed 29 Dec 2015.
119. Top 25 Pharma Companies by Global Sales. 2015 [cited 29 Dec 2015]. In PMLiVE Website [internet]. PMGroup Worldwide Ltd. Available: http://www.pmlive.com/top_pharma_list/global_revenues. Accessed 29 Dec 2015.
120. Drugs. 29 Dec 2015 [cited 29 Dec 2015]. In: U.S. Food and Drug Administration Website [internet]. Silver Spring: U.S. Food and Drug Administration. Available: http://www.fda.gov/Drugs/default.htm. Accessed 29 Dec 2015.
121. The University of Chicago Medicine. 2015 [cited 29 Dec 2015]. University of Chicago Hospitals Website [internet]. Chicago: The University of Chicago Medical Center. Available: http://www.uchospitals.edu/. Accessed 29 Dec 2015.
122. Walgreens. 2015 [cited 29 Dec 2015]. In: Walgreens Company Website [internet]. Deerfield: Walgreen Co. Available: http://www.walgreens.com/. Accessed 29 Dec 2015.
123. Learn About Glu. 2015 [cited 29 Dec 2015]. In: T1D Exchange for Type 1 Diabetes Website [internet]. Available: https://myglu.org/. Accessed 29 Dec 2015.
124. Zhongfei, Z., Ruofei, Z. Multimedia Data Mining – A Systematic Introduction to Concepts and Theory. Boca Raton: Taylor & Francis Group/CRC Press; 2008.
125. Bo L., Zhongfei, Z., Philip, S. Y. Relational data clustering: models, algorithms, and applications. Boca Raton: Taylor & Francis/CRC Press; 2010.
126. Zhen G., Zhongfei Z., Shenghuo, Z., Yun, C., Yihong, G. A two-level topic model towards knowledge discovery from citation networks, ieee transactions on knowledge and data engineering. IEEE Computer Society Press 2014; 26(4): 780 – 794.
127. GHI Spreadsheet ORS v63. In: Open Science Framework Website [internet]. Center for Open Science; 2015. Available: https://osf.io/zghvy/. Accessed 1 Jan 2016.
128. Awards and Recognition. 11 Dec 2015 [cited 29 Dec 2015]. In: Sanofi Company Website [internet]. Sanofi. Available: http://www.sanofi.us/l/us/en/layout.jsp?scat=B4A58C45-AA7A-4526-84A4-6E26FED0C640. Accessed 29 Dec 2015.
129. World Health Organization and World Bank. Monitoring Progress towards Universal Health Coverage at Country and Global Levels: A Framework. 2013; 1-10.
130. United Nations (UN). Sustainable Development Goals. 2015. http://www.un.org/sustainabledevelopment/sustainable-development-goals/.
131. Walker, N., Tam, Y., and Ingrid K Friberg. Overview of the Lives Saved Tool (LiST). BMC Public Health 2013;13 (Suppl 3): S1.
132. Gostin L. Global health law. Cambridge: Harvard University Press; 2014.
133. High-level panel of eminent persons on the post-2015 development. Bali Communiqué of the High-Level Panel, March 28, 2013.